Publications
042. Lead Optimization of Butyrolactone I as an Orally Bioavailable Antiallergic Agent Targeting FcγRIIB
Xie, C.-H.+; Xiao, H.-X.+; Song, P.-F.+; Liu, Q.-M.+; Wei, H.; Wu, L.; Zhu, G.-H.; Liu, G.-M.;* Zhang, Y.;* Wang, P.;* Yang, X.-W.*
J. Med. Chem. 2024, 67, 7504–7515
+ These authors contributed equally to this work.

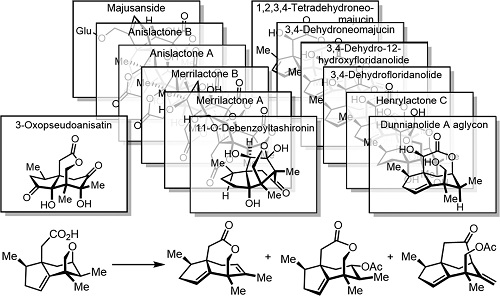
040. Divergent Total Syntheses of Illicium Sesquiterpenes through Late-Stage Skeletal Reorganization
Fu, P.+; Liu, T.+; Shen, Y.+; Lei, X.; Xiao, T.; Chen, P.; Qiu, D.; Wang, Z.; Zhang, Y.*
J. Am. Chem. Soc. 2023, 145, 18642–18648.
+ These authors contributed equally to this work.
038. Synthesis of Clionastatins A and B through Enhancement of Chlorination and Oxidation Levels of Testosterone
Cui, H.+; Shen, Y.+; Wang, R.; Wei, H.; Lei, X.; Chen, Y.; Fu, P.; Wang, H.; Bi, R.; Zhang, Y.*
Chin. J. Chem. 2022, 40, 2747–2755.
+ These authors contributed equally to this work.

037. Two-Stage Syntheses of Clionastatins A and B
Cui, H.+; Shen, Y.+; Chen, Y.; Wang, R.; Wei, H.; Fu, P.; Lei, X.; Wang, H.; Bi, R.; Zhang, Y.*
J. Am. Chem. Soc. 2022, 144, 8938–8944.
+ These authors contributed equally to this work.

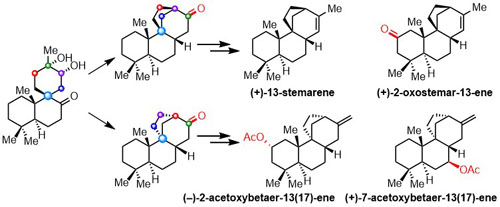
036. Total Synthesis of Stemarene and Betaerene Diterpenoids: Divergent Ring-Formation Strategy and Late-Stage C−H Functionalization
Chen, R.+; Zhang, F.+; Hua, Y.+; Shi, D.; Lei, X.; Xiao, H.; Wang, Y.; Zhang, Y.*
CCS Chem. 2022, 4, 987–995.
+ These authors contributed equally to this work.
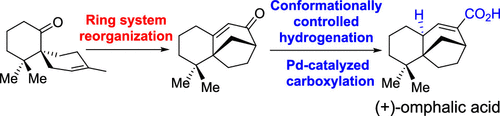
035. Total Synthesis and Assignment of the Absolute Configuration of (+)-Omphalic Acid
Chen, R.; Qiu, D.; Lei, X.; Niu, Y.; Hua, Y.; Peng, H.; Zeng, T.; Zhang, Y.*
Org. Lett. 2021, 23, 6972–6976.
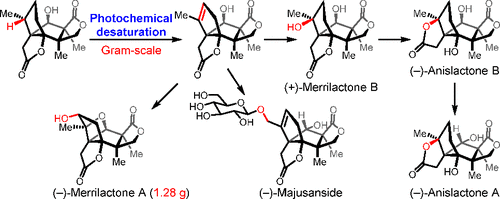
034. Site-Specific Photochemical Desaturation Enables Divergent Syntheses of Illicium Sesquiterpenes
Shen, Y.+; Li, L.+; Xiao, X.+; Yang, S.; Hua, Y.; Wang, Y.; Zhang, Y.-w.;* Zhang, Y.*
J. Am. Chem. Soc. 2021, 143, 3256–3263.
+ These authors contributed equally to this work.
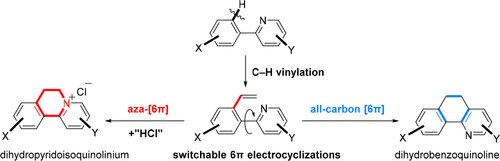
031. Merging C–H Vinylation with Switchable 6π-Electrocyclizations for Divergent Heterocycle Synthesis
Jiang, X.+; Zeng, Z.+; Hua, Y.+; Xu, B.; Shen, Y.; Xiong, J.; Qiu, H.; Wu, Y.; Hu, T.;* Zhang, Y.*
J. Am. Chem. Soc. 2020, 142, 15585–15594.
+ These authors contributed equally to this work.
030. Structure-Activity Relationship Study Enables the Discovery of a Novel Berberine Analogue as RXRα Activator to Inhibit Colon Cancer
Xu, B.+; Jiang, X.+; Xiong, J.; Lan, J.; Tian, Y.; Zhong, L.; Wang, X.; Xu, N.; Cao, H.; Zhang, W.; Zhang, H.; Hong, X.; Zhan, Y.-y.;* Zhang, Y.;* Hu, T.*
J. Med. Chem. 2020, 63, 5841–5855.
+ These authors contributed equally to this work.

027. Nesteretal A, A Novel Class of Cage-Like Polyketide from Marine-Derived Actinomycete Nesterenkonia halobia
Xie, C.-L.; Chen, R.; Yang, S.; Xia, J.-M.; Zhang, G.-Y.; Chen, C.-H.; Zhang, Y.; Yang, X.-W.*
Org. Lett. 2019, 21, 8174–8177.
021. Conformational Bias by a Removable Silyl Group: Construction of Bicyclo[n.3.1]alkenes by Ring Closing Metathesis
Lin, M.; Cai, P.-J.; Zeng, Z.; Lin, N.; Shen, Y.; Tang, B.; Li, F.; Chen, C.; Yu, Z.-X.;* Zhang, Y.*
Chem. Eur. J. 2018, 24, 2334–2338.

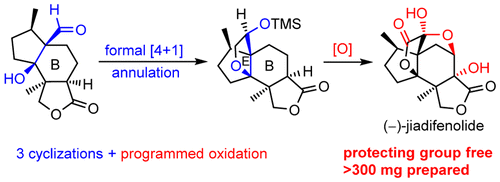
015. Protecting-Group-Free Total Synthesis of (−)-Jiadifenolide: Development of a [4 + 1] Annulation toward Multisubstituted Tetrahydrofurans
Shen, Y.; Li, L.; Pan, Z.; Wang, Y.; Li, J.; Wang, K.; Wang, X.; Zhang, Y.; Hu, T.; Zhang, Y.*
Org. Lett. 2015, 17, 5480–5483.
Top 20 Most Read Article in 2016; highlighted by Synfacts
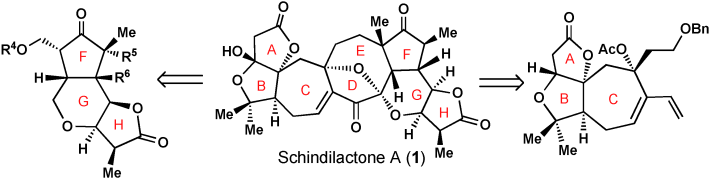
013. Diastereoselective Total Synthesis of (±)-Schindilactone A, Part 1: Construction of the ABC and FGH Ring Systems and Initial Attempts to Construct the CDEF Ring System
Sun, T.; Ren, W.; Xiao, Q.; Tang, Y.; Zhang, Y.; Li, Y.; ,Meng, F.; Liu, Y.; Zhao, M.; Xu, L.; Chen, J.; Yang, Z.
Chem. Asian J. 2012, 7, 2321–2333.
012. Multifaceted cytoprotection by synthetic polyacetylenes inspired by the ginseng-derived natural product, panaxytriol
Chou, T.; Dong, H.; Zhang, X.; Lei, X.; Hartung, J.; Zhang, Y.; Lee, J. H.; Danishefsky, S. J.
PNAS. 2011, 108, 14336–14341.
008. Stereoselective Construction of an Unprecedented 7−8 Fused Ring System in Micrandilactone A by [3,3]-Sigmatropic Rearrangement
Zhang, Y. ; Ren, W.; Lan, Y.; Xiao, Q.; Wang, K.; Xu, J.; Chen, J.; Yang, Z.
Org. Lett., 2008, 10, 665–668.

006. Thioureas as Ligands in the Pd-Catalyzed Intramolecular Pauson−Khand Reaction
Tang, Y.; Zhang, Y. ; Dai, M.; Luo, T.; Deng, L.; Chen, J.; Yang, Z.
Org. Lett., 2005, 7, 885–888.

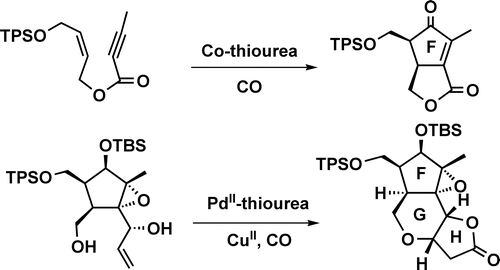
005. A Highly Efficient Synthesis of the FGH Ring of Micrandilactone A. Application of Thioureas as Ligands in the Co-catalyzed Pauson−Khand Reaction and Pd-Catalyzed Carbonylative Annulation
Tang, Y.; Zhang, Y. ; Dai, M.; Luo, T.; Deng, L.; Chen, J.; Yang, Z.
Org. Lett., 2005, 7, 885–888.
002. Synthesis of Asymmetric Propanetriol Analogues
Guo, Y.; Chen, J.; Fu, B.; Wang, N.; Jin, S.; Chen, L.; Zou, F.; Zhang, X.; Zhang, Y.; You, Z.
Chin. Chem. Lett. 2002, 13, 811–813.
|
|

